Abstract
Introduction: DLBCL is the most common subtype of non-Hodgkin lymphoma. R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) is established as the standard of care for patients (pts) with previously untreated DLBCL, but ~40% of pts will eventually relapse. For relapsed/refractory pts who are ineligible for transplant, clinical guidelines propose a broad spectrum of therapeutic options. However, little is known about treatment patterns and outcomes associated with 2L therapy in routine practice, particularly for pts less suitable for intensive therapy. Therefore, using real-world data, we evaluated 2L treatment patterns in DLBCL pts and overall survival (OS) in those pts who received 2L R-Benda or R-GemOx. We focused on these 2 treatments as they are typically used in the non-transplant setting in pts less suitable for aggressive therapy, and can typically be administered in an outpatient setting.
Methods: DLBCL pts receiving care from the US Veterans Health Administration were identified through their electronic medical records and raw oncology domain. Pts diagnosed with DLBCL (and no prior other types of malignancies) between 2004-2016, with ≥1-month follow-up and who received 2L treatment were included. OS (defined as time from the start of 2L therapy until death) was analyzed in pts who received 2L R-Benda or R-GemOx using the Kaplan-Meier method. Surviving pts were censored at data cutoff (December 31, 2017). Univariate and multivariate Cox regression analyses were undertaken to assess the impact of 2L treatment (in particular, R-GemOx vs R-Benda) on OS.
Results: A total of 2600 DLBCL pts were identified: 2039 received 1L and 702 received 1L and 2L therapy. Among the 702 pts treated with 2L therapy, regimens included R-ICE (n=77; 11.0%), R-CHOP (n=75; 10.7%), rituximab monotherapy (n=34; 4.8%), R-Benda (n=32; 4.6%), methotrexate (n=24; 3.4%), R-ESHAP (n=23; 3.3%), R-DHAP/R-EPOCH/R-GDP (n=18; 2.6%), rituximab plus cyclophosphamide-doxorubicin-vinblastine-vincristine (n=14; 2.0%), R-CVP (n=11; 1.6%), rituximab plus cyclophosphamide-etoposide-vincristine (n=11; 1.6%), and R-GemOx (n=10; 1.4%). Of the remaining pts, 267 (38.0%) received regimens with agent(s) included in the NCCN guidelines, while 106 (15.1%) received regimens with at least 1 agent not guideline-recommended.
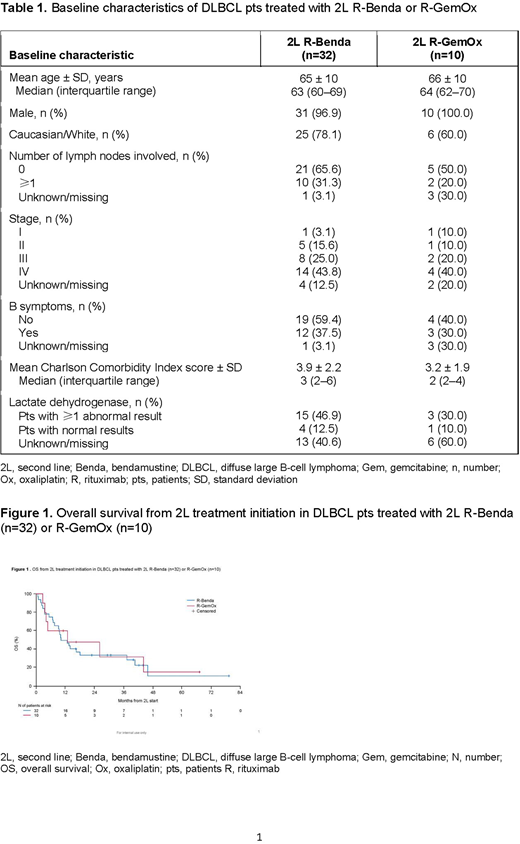
Baseline characteristics for pts treated with 2L R-Benda (n=32) or R-GemOx (n=10) are shown in Table 1. There was an imbalance between the 2 cohorts with regard to race, number of involved lymph nodes, B symptoms, Charlson Comorbidity Index score, and abnormal lactate dehydrogenase results. After 24 deaths in the R-Benda cohort and 7 deaths in the R-GemOx cohort, median OS was estimated at 11 and 13 months, respectively (Figure 1). Median follow-up time after start of 2L treatment was 11.3 and 11.7 months, respectively. The Kaplan-Meier curves of the 2 cohorts overlapped at multiple timepoints during follow-up. Respective 1-year OS rates (95% confidence interval [CI]) with R-Benda and R-GemOx were 50.0% (31.9%, 65.7%) and 60.0% (25.3%, 82.7%). Compared with R-Benda, R-GemOx did not significantly predict longer OS in either the univariate (hazard ratio [HR]: 0.94; 95% CI: 0.41, 2.19; p=0.893) or multivariate (HR: 1.07; 95% CI: 0.46, 2.50; p=0.873) analyses.
Conclusions: This real-world study highlights the diversity of 2L treatment regimens used in DLBCL pts. There was no apparent difference in OS between R-Benda- and R-GemOx-treated pts and, with a median OS of approximately 1 year after 2L initiation with either regimen, there is clearly an unmet need in this setting. The main limitation of the study relates to the small sample size of each treatment cohort. Further research using other real-world data sources is warranted.
Ionescu-Ittu:Analysis Group, Inc.: Employment; F. Hoffman-La Roche Ltd: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Roche for the conduct of this study. Shang:F. Hoffmann-La Roche Ltd.: Employment, Other: Ownership interests non-PLC. Guérin:F. Hoffman-La Roche Ltd: Other: I am an employee of Analysis Group, Inc., which received consulting fees from Roche for the conduct of this study; Analysis Group, Inc.: Employment. Shi:F. Hoffman-La Roche Ltd: Research Funding; Bravo4Health: Other: Ownership interests non-PLC; Genentech: Research Funding; Chiasma: Research Funding; Intuitive Surgical: Consultancy. Shi:F. Hoffman-La Roche Ltd: Other: I am an employee of Analysis Group, Inc., which received consulting fees from Roche for the conduct of this study; Analysis Group, Inc.: Employment. Qayum:F. Hoffmann-La Roche Ltd: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal